BENDEKA is indicated for the treatment of patients with chronic lymphocytic leukemia (CLL) — efficacy relative to first-line therapies other than chlorambucil has not been established — or patients with indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen.
Offer your patients a short, fixed course of therapy with BENDEKA1
Patients have time off treatment after infusing1:
On Day 1 and Day 2 at 100 mg/m2 administered intravenously

On Day 1 and Day 2 at 120 mg/m2 administered intravenously
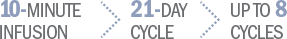
INDICATIONS
BENDEKA is indicated for the treatment of patients with
- Chronic lymphocytic leukemia (CLL). Efficacy relative to first-line therapies other than chlorambucil has not been established.
- Indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen.
Important Safety Information
Contraindication: BENDEKA is contraindicated in patients with a known hypersensitivity (e.g., anaphylactic and anaphylactoid reactions) to bendamustine, polyethylene glycol 400, propylene glycol, or monothioglycerol.
Myelosuppression: Bendamustine HCl caused severe myelosuppression (Grade 3-4) in 98% of patients in the two NHL studies. Three patients (2%) died from myelosuppression-related adverse reactions. BENDEKA causes myelosuppression. Monitor leukocytes, platelets, hemoglobin (Hgb), and neutrophils frequently. Myelosuppression may require dose delays and/or subsequent dose reductions if recovery to the recommended values has not occurred by the first day of the next scheduled cycle.
Infections: Infection, including pneumonia, sepsis, septic shock, hepatitis and death has occurred. Patients with myelosuppression following treatment with BENDEKA are more susceptible to infections. Patients treated with BENDEKA are at risk for reactivation of infections including (but not limited to) hepatitis B, cytomegalovirus, Mycobacterium tuberculosis, and herpes zoster. Patients should undergo appropriate monitoring, prophylaxis, and treatment measures prior to administration.
Progressive Multifocal Leukoencephalopathy (PML): PML including fatal cases, have occurred following treatment with bendamustine, primarily in combination with rituximab or obinutuzumab. Consider PML in the differential diagnosis in patients with new or worsening neurological, cognitive or behavioral signs or symptoms. If PML is suspected, withhold BENDEKA treatment and perform appropriate diagnostic evaluations. Consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
Anaphylaxis and Infusion Reactions: Infusion reactions to bendamustine HCl have occurred commonly in clinical trials. Symptoms include fever, chills, pruritus, and rash. In rare instances severe anaphylactic and anaphylactoid reactions have occurred, particularly in the second and subsequent cycles of therapy. Monitor clinically and discontinue drug for severe reactions. Ask patients about symptoms suggestive of infusion reactions after their first cycle of therapy. Patients who experienced Grade 3 or worse allergic-type reactions were not typically rechallenged. Consider measures to prevent severe reactions, including antihistamines, antipyretics, and corticosteroids in subsequent cycles in patients who have experienced Grade 1 or 2 infusion reactions. Discontinue BENDEKA for patients with Grade 4 reactions. Consider discontinuation for Grade 3 infusion reactions as clinically appropriate considering individual benefits, risks, and supportive care.
Tumor Lysis Syndrome: Tumor lysis syndrome associated with bendamustine HCl has occurred. The onset tends to be within the first treatment cycle with bendamustine HCl and, without intervention, may lead to acute renal failure and death. Preventive measures include vigorous hydration and close monitoring of blood chemistry, particularly potassium and uric acid levels. There may be an increased risk of severe skin toxicity when bendamustine HCl and allopurinol are administered concomitantly.
Skin Reactions: Fatal and serious skin reactions have been reported with bendamustine HCl and include, toxic skin reactions, [Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS)], bullous exanthema and rash. Events occurred when bendamustine HCl was given as a single agent and in combination with other anticancer agents or allopurinol. Where skin reactions occur, they may be progressive and increase in severity with further treatment. Monitor patients with skin reactions closely. If skin reactions are severe or progressive, withhold or discontinue BENDEKA.
Hepatotoxicity: Fatal and serious cases of liver injury have been reported with bendamustine HCl. Combination therapy, progressive disease or reactivation of hepatitis B were confounding factors in some patients. Most cases were reported within the first three months of starting therapy. Monitor liver chemistry tests prior to and during BENDEKA therapy.
Other Malignancies: There are reports of pre-malignant and malignant diseases that have developed in patients, who have been treated with bendamustine HCl, including myelodysplastic syndrome, myeloproliferative disorders, acute myeloid leukemia, bronchial carcinoma and non-melanoma skin cancer, including basal cell carcinoma and squamous cell carcinoma. Monitor patients for the development of secondary malignancies. Perform dermatologic evaluations during and after treatment with BENDEKA.
Extravasation Injury: Bendamustine HCl extravasations have been reported in post-marketing resulting in hospitalizations from erythema, marked swelling, and pain. Assure good venous access prior to starting BENDEKA infusion and monitor the intravenous infusion site for redness, swelling, pain, infection, and necrosis during and after administration of BENDEKA.
Embryo-Fetal Toxicity: BENDEKA can cause fetal harm when administered to a pregnant woman. Conduct pregnancy testing prior to initiating treatment and advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use an effective method of contraception during treatment with BENDEKA and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with BENDEKA and for 3 months after the last dose.
Lactation: Advise patients that breastfeeding is not recommended during treatment with BENDEKA, and for 1 week after the last dose.
Most Common Adverse Reactions:
- Adverse reactions (frequency >5%) during infusion and within 24 hours post-infusion are nausea and fatigue.
- Most common adverse reactions for CLL (frequency ≥15%) are anemia, thrombocytopenia, neutropenia, lymphopenia, leukopenia, hyperbilirubinemia, pyrexia, nausea, and vomiting
- Most common adverse reactions for NHL (frequency ≥15%) are lymphopenia, leukopenia, anemia neutropenia, thrombocytopenia, nausea, fatigue, vomiting, diarrhea, pyrexia, constipation, anorexia, cough, headache, weight decreased, dyspnea, rash, and stomatitis
Please see Full Prescribing Information for BENDEKA
INDICATIONS
BENDEKA is indicated for the treatment of patients with
- Chronic lymphocytic leukemia (CLL). Efficacy relative to first-line therapies other than chlorambucil has not been established.
- Indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen.
Important Safety Information
Contraindication: BENDEKA is contraindicated in patients with a known hypersensitivity (e.g., anaphylactic and anaphylactoid reactions) to bendamustine, polyethylene glycol 400, propylene glycol, or monothioglycerol.
Myelosuppression: Bendamustine HCl caused severe myelosuppression (Grade 3-4) in 98% of patients in the two NHL studies. Three patients (2%) died from myelosuppression-related adverse reactions. BENDEKA causes myelosuppression. Monitor leukocytes, platelets, hemoglobin (Hgb), and neutrophils frequently. Myelosuppression may require dose delays and/or subsequent dose reductions if recovery to the recommended values has not occurred by the first day of the next scheduled cycle.
Infections: Infection, including pneumonia, sepsis, septic shock, hepatitis and death has occurred. Patients with myelosuppression following treatment with BENDEKA are more susceptible to infections. Patients treated with BENDEKA are at risk for reactivation of infections including (but not limited to) hepatitis B, cytomegalovirus, Mycobacterium tuberculosis, and herpes zoster. Patients should undergo appropriate monitoring, prophylaxis, and treatment measures prior to administration.
Progressive Multifocal Leukoencephalopathy (PML): PML including fatal cases, have occurred following treatment with bendamustine, primarily in combination with rituximab or obinutuzumab. Consider PML in the differential diagnosis in patients with new or worsening neurological, cognitive or behavioral signs or symptoms. If PML is suspected, withhold BENDEKA treatment and perform appropriate diagnostic evaluations. Consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
Anaphylaxis and Infusion Reactions: Infusion reactions to bendamustine HCl have occurred commonly in clinical trials. Symptoms include fever, chills, pruritus, and rash. In rare instances severe anaphylactic and anaphylactoid reactions have occurred, particularly in the second and subsequent cycles of therapy. Monitor clinically and discontinue drug for severe (Grade 3-4) reactions. Ask patients about symptoms suggestive of infusion reactions after their first cycle of therapy. Consider measures to prevent severe reactions, including antihistamines, antipyretics, and corticosteroids in subsequent cycles in patients who have experienced Grade 1 or 2 infusion reactions.
Tumor Lysis Syndrome: Tumor lysis syndrome associated with bendamustine HCl has occurred. The onset tends to be within the first treatment cycle with bendamustine HCl and, without intervention, may lead to acute renal failure and death. Preventive measures include vigorous hydration and close monitoring of blood chemistry, particularly potassium and uric acid levels. There may be an increased risk of severe skin toxicity when bendamustine HCl and allopurinol are administered concomitantly.
Skin Reactions: Fatal and serious skin reactions have been reported with bendamustine HCl and include, toxic skin reactions, [Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS)], bullous exanthema and rash. Events occurred when bendamustine HCl was given as a single agent and in combination with other anticancer agents or allopurinol. Where skin reactions occur, they may be progressive and increase in severity with further treatment. Monitor patients with skin reactions closely. If skin reactions are severe or progressive, withhold or discontinue BENDEKA.
Hepatotoxicity: Fatal and serious cases of liver injury have been reported with bendamustine HCl. Combination therapy, progressive disease or reactivation of hepatitis B were confounding factors in some patients. Most cases were reported within the first three months of starting therapy. Monitor liver chemistry tests prior to and during BENDEKA therapy.
Other Malignancies: There are reports of pre-malignant and malignant diseases that have developed in patients, who have been treated with bendamustine HCl, including myelodysplastic syndrome, myeloproliferative disorders, acute myeloid leukemia, bronchial carcinoma and non-melanoma skin cancer, including basal cell carcinoma and squamous cell carcinoma. Monitor patients for the development of secondary malignancies. Perform dermatologic evaluations during and after treatment with BENDEKA.
Extravasation Injury: Bendamustine HCl extravasations have been reported in post-marketing resulting in hospitalizations from erythema, marked swelling, and pain. Assure good venous access prior to starting BENDEKA infusion and monitor the intravenous infusion site for redness, swelling, pain, infection, and necrosis during and after administration of BENDEKA.
Embryo-Fetal Toxicity: BENDEKA can cause fetal harm when administered to a pregnant woman. Conduct pregnancy testing prior to initiating treatment and advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use an effective method of contraception during treatment with BENDEKA and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with BENDEKA and for 3 months after the last dose.
Lactation: Advise patients that breastfeeding is not recommended during treatment with BENDEKA, and for 1 week after the last dose.
Most Common Adverse Reactions:
- Adverse reactions (frequency >5%) during infusion and within 24 hours post-infusion are nausea and fatigue.
- Most common adverse reactions for CLL (frequency ≥15%) are anemia, thrombocytopenia, neutropenia, lymphopenia, leukopenia, hyperbilirubinemia, pyrexia, nausea, and vomiting
- Most common adverse reactions for NHL (frequency ≥15%) are lymphopenia, leukopenia, anemia neutropenia, thrombocytopenia, nausea, fatigue, vomiting, diarrhea, pyrexia, constipation, anorexia, cough, headache, weight decreased, dyspnea, rash, and stomatitis
Please see Full Prescribing Information for BENDEKA
Reference: 1. BENDEKA® (bendamustine hydrochloride) injection [Current Prescribing Information]. Parsippany, NJ: Teva Pharmaceuticals.



